TMS Therapy

NeuroStar TMS Therapy is a new treatment cleared by the US Food and Drug Administration (FDA) for patients suffering from depression* who have not achieved satisfactory improvement from prior antidepressant treatment. TMS stands for "transcranial magnetic stimulation."
TMS Therapy is a treatment that can be performed in a psychiatrist's office, under their supervision, using a medical device called the NeuroStar TMS Therapy system. NeuroStar TMS Therapy is:
· Non-invasive, meaning that it does not involve surgery. It does not require any anesthesia or sedation, as the patient remains awake and alert during the treatment.
· Non-systemic, meaning that it is not taken by mouth and does not circulate in the blood stream throughout the body.
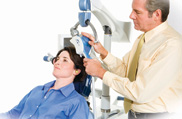 The typical initial treatment course consists of 5 treatments per week over a 4-6 week period, for an average of 20-30 total treatments. Each treatment session lasts approximately 40 minutes.
The typical initial treatment course consists of 5 treatments per week over a 4-6 week period, for an average of 20-30 total treatments. Each treatment session lasts approximately 40 minutes.
* NeuroStar TMS Therapy® is indicated for the treatment of Major Depressive
Disorder in adult patients who have failed to achieve satisfactory improvement
from one prior antidepressant medication at or above the minimal effective
dose and duration in the current episode.
